National
U.S. health officials expand approach to monkeypox vaccines as cases crest
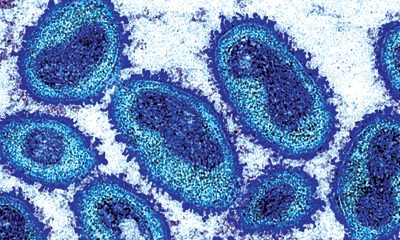
At the end of a summer when the number of cases in the monkeypox outbreak rose sharply, the increase in reported infections now appears to be cresting amid increased public messaging and access to vaccines, prompting U.S. health officials to expand their strategy with a new equity-based effort to combat the disease.
Although the reported number of cases, according to most data from the Centers for Disease Control & Prevention, has reached 18,417 in the United States, the number of additional cases decreased from the high at the start of the month, suggesting a downward trajectory in the spread of the disease as vaccines become more readily available.
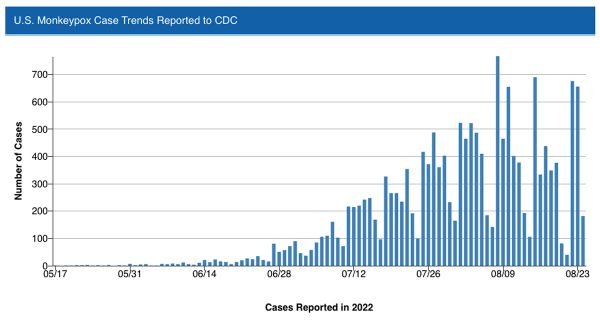
The numbers are also consistent with a new study finding a significant number of gay and bisexual men, as well as other men who have sex with men, have been limiting contact with casual sex partners, which has been the driving force in the spread of monkeypox. The report from the CDC last week found limiting one-time sexual encounters can significantly reduce the transmission of monkeypox virus, while about half of men who have sex with men are cutting down on sexual activity amid the outbreak, including one-night stands and app hookups.
With the trajectory of monkeypox on the decline, the Biden administration announced a new initiative with the goal of ensuring vaccine distribution is consistent with the value of equity, including on the basis of geographic, racial, and ethnic lines. A total of 10,000 doses of vaccines in the federal government’s supply will be earmarked for localities that have used 50 percent of their allocated supply to support equity interventions, such as outreach to Black and Latino communities, which have been disproportionately affected by the disease or a specific event and celebration for LGBTQ people, health officials announced Tuesday.
Demetre Daskalakis, the Biden administration’s face of LGBTQ outreach for monkeypox and deputy coordinator for the White House monkeypox task force, laid out the details for the new equity-based supplementary initiative in a conference call Tuesday with reporters.
“So what we mean by an equity intervention is what works in your state, county, or city to reach people who we may not be reaching, especially people of color and members of the LGBTQI+ population,” Daskalakis said. “What it means is: It can be working with a specific group or venue that reaches the right people for monkeypox prevention. Once these innovative strategies have been reviewed by CDC, vaccines will be supplied to jumpstart these ideas and accelerate reach deeper into communities.”
The additional equity-based approach to monkeypox vaccine distribution is consistent with the Biden administration’s efforts in recent weeks to distribute additional shots to localities hosting large-scale events for LGTBQ people at the end of the summer, such as Black Pride in Atlanta and Southern Decadence in New Orleans.
Louisiana Gov. John Bel Edwards joined the conference call with reporters on Tuesday and had high praise for the Biden administration for making the additional 6,000 doses of monkeypox vaccine available in time for Southern Decadence, which takes place in the final week of August through Labor Day weekend.
“This is an example — I think a really solid example — of what a federal-state-local partnership and — and then the community providers as well,” Bel Edwards said. “Because the public health folks in New Orleans have been tremendous, but also the community providers.”
Bel Edwards said health officials in the Biden administration have, in addition to providing more vaccines, sent down multidisciplinary teams to New Orleans to help the state organize and prepare as well as set up testing and vaccination sites “that are going to be convenient for the at-risk population.”
A reporter from the New Orleans Advocate on the conference call, however, asked a pointed question on the recent distribution of vaccines to New Orleans in advance of Southern Decadence: The current approach to vaccine administration requires a series of shots, and even with new distribution most people won’t have even had their second shot by that time, so how can Southern Decadence think they will be protected, especially when vaccines take time to become fully effective?
Daskalakis, while promoting the equity-based approach to vaccine distribution, said the Biden administration has been “very clear” that first shot of the monkeypox vaccine “doesn’t mean that you’re protected for the event.”
“We’re going to talk to them about lots of other strategies that they can reduce risk of acquiring monkeypox, but also make it clear that that shot is not for today; it’s for four weeks from now, plus two weeks after that second dose when you get maximum protection,” Daskalakis said.
First death of monkeypox patient reported
Although the number of cases is cresting, concern about monkeypox continues as well as the potential danger of the disease. Case in point: The death of a hospital patient in Texas who had monkeypox, but may have to succumbed to other factors, has drawn attention amid a conventional understanding the skin disease isn’t fatal. The case represents the first time in the United States that a patient with monkeypox died while having the condition.
The patient, as confirmed by the Texas Department of State Health Services on Tuesday, was an adult resident of Harris County who was “severely immunocompromised” and state health officials reviewing the case said it is under investigation to determine what role monkeypox played in the death.
Jenny McQuiston, a CDC official who specializes in research on zoological diseases that spread from animals to people, said in response to a question on the casualty that health officials are also evaluating the death and the role monkeypox played.
“I think it’s important to emphasize that deaths due to monkeypox, while possible, remain very rare,” McQuiston said. “In most cases, people are experiencing infection that resolves over time. And there have been very few deaths even recorded globally. Out of over 40,000 cases around the world, only a handful of fatalities have been reported.”
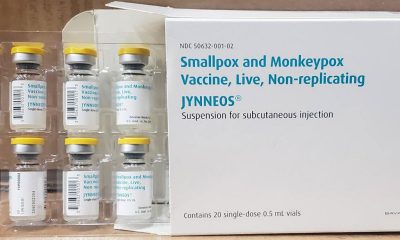
Despite the cresting in the number of cases, many health experts aren’t sold on the new approach to vaccines announced earlier this month by the Biden administration, which sought to expand existing doses of vaccines fivefold as supply hasn’t met demand. The new vaccine approach calls for injecting the JYNNEOS vaccine from the subcutaneous route (delivery of the vaccine under the fat layer underneath the skin) to the intradermal route (delivery of the vaccine into the layer of skin just underneath the top layer).
Bob Fenton, a regional administrator for FEMA and the response coordinator for the White House task force, said about 75 percent of jurisdictions have already adopted the new approach to vaccine injection, while an additional 20 percent are working toward a “fully operational intradermal method.”
“We continue to be laser-focused on doing everything within our power to help jurisdictions and clinicians get shots in arms,” Fenton said. “We’re seeing more and more jurisdictions adopt the intradermal administration.”
Data of this new intradermal approach, critics have said, is insufficient to support the idea it will be as effective as subcutaneous injections, although the Biden administration continues to give assurances the new route for injections is tested and safe. According to a report earlier this month in the Washington Post, the manufacturer of the JYNNEOS vaccine in Denmark, Bavaria Nordic, privately threatened to cut off supply of the shots based on a conversation with health officials on objections the vaccine hasn’t been approved for intradermal use.
McQuiston, in response to a question on whether or not U.S. health officials are collecting newly available real-world information on the results of the new vaccine approach, said U.S. health officials continue to receive data on monkeypox and soon onboard information from additional states.
“CDC operates a system called VAERS — or the Vaccine Adverse Event Reporting System — and we’re actively looking at…different types of events that might be reported post-vaccination,” McQuiston said. ” And we are actively gathering information from the different jurisdictions and states and cities about which vaccines they’re administering — whether it’s subcutaneous or intradermal — and we are gathering those data now, as we speak.”

The Comings & Goings column is about sharing the professional successes of our community. We want to recognize those landing new jobs, new clients for their business, joining boards of organizations and other achievements. Please share your successes with us at [email protected].
Congratulations to Gil Pontes III on his recent appointment to the Financial Advisory Board for the City of Wilton Manors, Fla. Upon being appointed he said, “I’m honored to join the Financial Advisory Board for the City of Wilton Manors at such an important moment for our community. In my role as Executive Director of the NextGen Chamber of Commerce, I spend much of my time focused on economic growth, fiscal sustainability, and the long-term competitiveness of emerging business leaders. I look forward to bringing that perspective to Wilton Manors — helping ensure responsible stewardship of public resources while supporting a vibrant, inclusive local economy.”
Pontes is a nonprofit executive with years of development, operations, budget, management, and strategic planning experience in 501(c)(3), 501(c)(4), and political organizations. Pontes is currently executive director of NextGen, Chamber of Commerce. NextGen Chamber’s mission is to “empower emerging business leaders by generating insights, encouraging engagement, and nurturing leadership development to shape the future economy.” Prior to that he served as managing director of The Nora Project, and director of development also at The Nora Project. He has held a number of other positions including Major Gifts Officer, Thundermist Health Center, and has worked in both real estate and banking including as Business Solutions Adviser, Ironwood Financial. For three years he was a Selectman, Town of Berkley, Mass. In that role, he managed HR and general governance for town government. There were 200+ staff and 6,500 constituents. He balanced a $20,000,000 budget annually, established an Economic Development Committee, and hired the first town administrator.
Pontes earned his bachelor’s degree in political science from the University of Massachusetts, Dartmouth.
Kansas
ACLU sues Kansas over law invalidating trans residents’ IDs
A new Kansas bill requires transgender residents to have their driver’s licenses reflect their sex assigned at birth, invalidating current licenses.

Transgender people across Kansas received letters in the mail on Wednesday demanding the immediate surrender of their driver’s licenses following passage of one of the harshest transgender bathroom bans in the nation. Now the American Civil Liberties Union is filing a lawsuit to block the ban and protect transgender residents from what advocates describe as “sweeping” and “punitive” consequences.
Independent journalist Erin Reed broke the story Wednesday after lawmakers approved House Substitute for Senate Bill 244. In her reporting, Reed included a photo of the letter sent to transgender Kansans, requiring them to obtain a driver’s license that reflects their sex assigned at birth rather than the gender with which they identify.
According to the reporting, transgender Kansans must surrender their driver’s licenses and that their current credentials — regardless of expiration date — will be considered invalid upon the law’s publication. The move effectively nullifies previously issued identification documents, creating immediate uncertainty for those impacted.
House Substitute for Senate Bill 244 also stipulates that any transgender person caught driving without a valid license could face a class B misdemeanor, punishable by up to six months in jail and a $1,000 fine. That potential penalty adds a criminal dimension to what began as an administrative action. It also compounds the legal risks for transgender Kansans, as the state already requires county jails to house inmates according to sex assigned at birth — a policy that advocates say can place transgender detainees at heightened risk.
Beyond identification issues, SB 244 not only bans transgender people from using restrooms that match their gender identity in government buildings — including libraries, courthouses, state parks, hospitals, and interstate rest stops — with the possibility for criminal penalties, but also allows for what critics have described as a “bathroom bounty hunter” provision. The measure permits anyone who encounters a transgender person in a restroom — including potentially in private businesses — to sue them for large sums of money, dramatically expanding the scope of enforcement beyond government authorities.
The lawsuit challenging SB 244 was filed today in the District Court of Douglas County on behalf of anonymous plaintiffs Daniel Doe and Matthew Moe by the American Civil Liberties Union, the ACLU of Kansas, and Ballard Spahr LLP. The complaint argues that SB 244 violates the Kansas Constitution’s protections for personal autonomy, privacy, equality under the law, due process, and freedom of speech.
Additionally, the American Civil Liberties Union filed a temporary restraining order on behalf of the anonymous plaintiffs, arguing that the order — followed by a temporary injunction — is necessary to prevent the “irreparable harm” that would result from SB 244.
State Rep. Abi Boatman, a Wichita Democrat and the only transgender member of the Kansas Legislature, told the Kansas City Star on Wednesday that “persecution is the point.”
“This legislation is a direct attack on the dignity and humanity of transgender Kansans,” said Monica Bennett, legal director of the ACLU of Kansas. “It undermines our state’s strong constitutional protections against government overreach and persecution.”
“SB 244 is a cruel and craven threat to public safety all in the name of fostering fear, division, and paranoia,” said Harper Seldin, senior staff attorney for the ACLU’s LGBTQ & HIV Rights Project. “The invalidation of state-issued IDs threatens to out transgender people against their will every time they apply for a job, rent an apartment, or interact with police. Taken as a whole, SB 244 is a transparent attempt to deny transgender people autonomy over their own identities and push them out of public life altogether.”
“SB 244 presents a state-sanctioned attack on transgender people aimed at silencing, dehumanizing, and alienating Kansans whose gender identity does not conform to the state legislature’s preferences,” said Heather St. Clair, a Ballard Spahr litigator working on the case. “Ballard Spahr is committed to standing with the ACLU and the plaintiffs in fighting on behalf of transgender Kansans for a remedy against the injustices presented by SB 244, and is dedicated to protecting the constitutional rights jeopardized by this new law.”
National
After layoffs at Advocate, parent company acquires ‘Them’ from Conde Nast
Top editorial staff let go last week

Former staff members at the Advocate and Out magazines revealed that parent company Equalpride laid off a number of employees late last week.
Those let go included Advocate editor-in-chief Alex Cooper, Pride.com editor-in-chief Rachel Shatto, brand partnerships manager Erin Manley, community editor Marie-Adélina de la Ferriére, and Out magazine staff writers Moises Mendez and Bernardo Sim, according to a report in Hollywood Reporter.
Cooper, who joined the company in 2021, posted to social media that, “Few people have had the privilege of leading this legendary LGBTQ+ news outlet, and I’m deeply honored to have been one of them. To my team: thank you for the last four years. You’ve been the best. For those also affected today, please let me know how I can support you.”
The Advocate’s PR firm when reached by the Blade said it no longer represents the company. Emails to the Advocate went unanswered.
Equalpride on Friday announced it acquired “Them,” a digital LGBTQ outlet founded in 2017 by Conde Nast.
“Equalpride exists to elevate, celebrate and protect LGBTQ+ storytelling at scale,” Equalpride CEO Mark Berryhill said according to Hollywood Reporter. “By combining the strengths of our brands with this respected digital platform, we’re creating a unified ecosystem that delivers even more impact for our audiences, advertisers, and community partners.”
It’s not clear if “Them” staff would take over editorial responsibilities for the Advocate and Out.